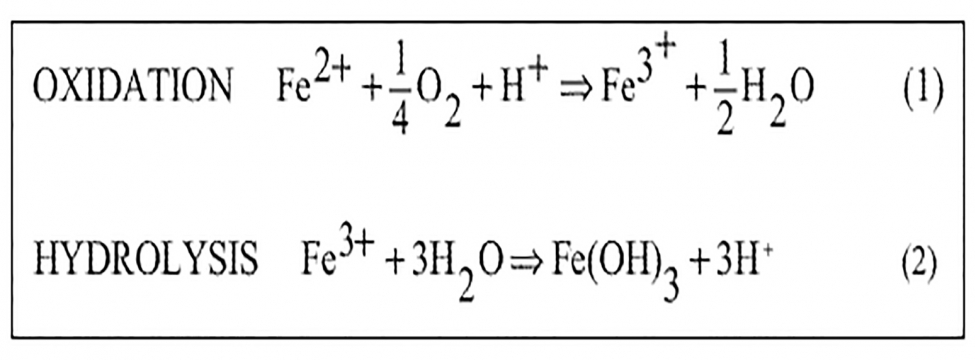During active mining and post closure, the control of water requires prudent site management to firstly minimise the generation of minewater and secondly to treat where necessary. In order to reduce associated treatment costs in the long term, artificial wetlands have gained acceptance, particularly for treating coal mine drainage. In such circumstances, where iron is often the predominant contaminant of concern, removal utilises the natural oxidation of iron II (Fe2+) to iron III (Fe3+) followed by hydrolysis of the iron II as shown below: As the hydrolysis process generates proton acidity (H+), the water being treated must contain sufficient alkalinity to buffer this reaction if it is to remain ‘net alkaline’. For this reason, waters with insufficient buffering capacity are termed ‘net acidic’ and require distinctly different treatment requirements.